An influential advisory group for centers for disease control and prevention voted Thursday to reduce existing recommendations for combined MMRV shot that protects against measles, papers, rubella and chickenpox.
The CDC Vaccine Advisory Committee, called ACIP, voted 8-3 to reduce recommendations. A person refrained from voting due to a conflict of interest.
Another vote to abandon a long -standing recommendation to give all newborns a hepatitis B vaccine at birth was delayed until Friday.
With respect to the MMRV vaccine, the vote of the advisors means that the combined shot, which contains measles, paper, rubella and chickenpox (chickenpox), all at once, will no longer be recommended so that younger children have their first dose about 12 months.
On the other hand, the Committee recommended that these children obtain two separate shots, one that combines measles/paper/rubella and a second shot for chickenpox. The combined take will continue to be recommended as an option for the second dose of a child, which occurs between the ages of 4 to 6.
The vote on the MMRV vaccine vaccine needs firmation of the director of the Interim CDC or the Secretary of Health and Human Services.
However, even with that vote, panel members seemed reluctant to do anything that could restrict access for 50% of American children covered through the Vaccine for Children (VFC) program financed by the federal government, voting against any change.
But HHS says that they still need to consider the result of the vote of the non -binding advisors, saying in a statement: “The HHS will examine all the implications of insurance coverage after the recommendation of ACIP today, before a final decision on the adoption of the interim director. An ACIP recommendation becomes part of the CD Immunization program if it is adopted by the director of CD.”
In simple terms, if the CDC accepts the group’s recommendations, parents of children who receive VFC vaccines, can still choose to give their children a combination of MMRV for their first dose if they do not want to separate the shots and both options, but others are only recommended to obtain a separate shot of MMR and chicken shots if they are less than 4 years for their first dome.
During the discussion before the vote, some members of the Committee said They were worried The proposed changes could eliminate parents’ elections, especially with respect to the combined MMRV vaccine.

The CDC Immunization Advisory Committee is observed during a meeting at the headquarters of the Centers for Disease Control and Prevention on September 18, 2025 in Atlanta, Georgia.
Elijah Nouvelage/Getty Images
Currently, depending on the previous recommendation, all parents have the option of giving their children less than 4 years of measles, paper, rubella and chickenpox, all as a single shot, or they can choose to separate the doses, give measles, papers and rubella as a shot and chickenpox as a separate dose at the same time. Some studies have suggested a slightly high risk, but in general very rare, of seizures when four are given as a combined shot for children from 12 to 15 months.
But many parents and clinics can still prefer a single shot. Children also get a second dose of MMRV after 4 years.
The committee was not considering eliminating or recommending against MMRV or hepatitis B vaccines completely. But the changes that have been proposed could result in large interruptions and more diseases, experts warned.
Experts say that these changes could also cause confusion, more medical appointments and more individual shots for children, which could lead to lost cases or more infections. It could also complicate vaccine supply and manufacturing logistics.
“The disadvantage of giving two doses, or as suggested, separating the two doses, is that we know that compliance falls, and the advantage of combined vaccines is that children and adults are more likely to complete the requirements of the vaccine if it is administered as a single dose,” said the member of the ACIP member, Dr. Cody Meissner.
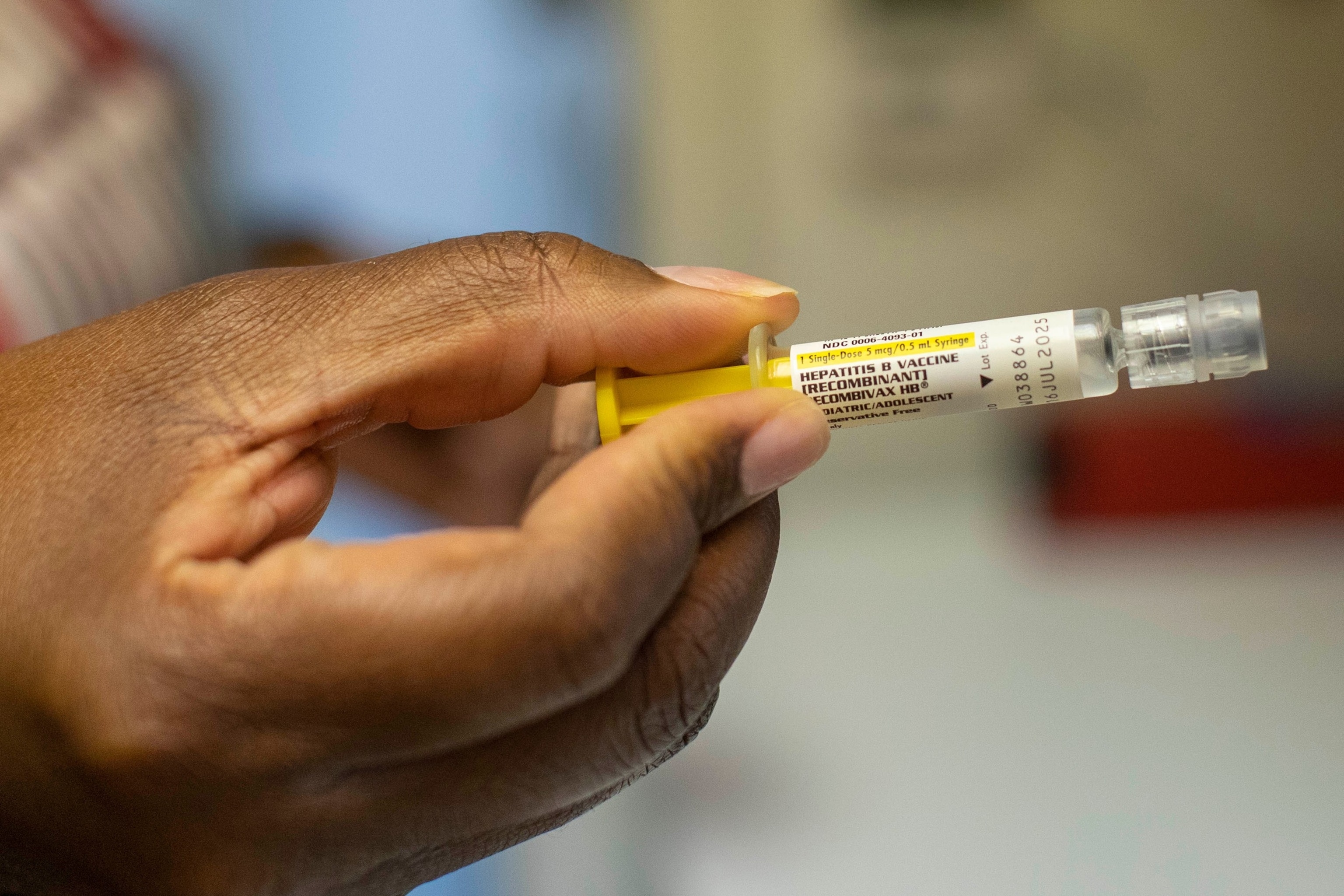
A hepatitis B vaccine in Atlanta, Georgia, on September 29, 2023.
Alyssa Pointer for The Washington Post through Getty Images
On Wednesday, Republican Senator Bill Cassidy, a liver doctor, told journalists that Americans should not trust the decisions of this committee if they make certain changes in the current vaccination hours.
“I can promise him that there will be some transmission of hepatitis B,” Cassidy told reporters when asked what would happen if the committee makes changes in that existing recommendation.
This week’s meeting is the second of the Committee since the Secretary of Health and Human Services, Robert F. Kennedy Jr., dismissed its 17 members in June. Of the 12 advisors who have been appointed since then, many have previously expressed vaccine views.
The majority of the main insurance suppliers have said that they will continue to cover existing vaccines at least until 2026. But many care about the access and impact of any change that may negatively affect the more than half of the US children who receive free shots financed through a federal program, which is linked to the recommendations of the CDC committee.




